| HOME | | | Japanese | | | Profile and Publication List |

Language: English | Japanese
Chem. Sci. 2024, 15, 1953; Chem. Sci. 2022, 13, 3140; ACS Appl. Mater. Interfaces 2021, 13, 38613
 |
Toward Three-dimensionally Ordered Nanoporous Graphene Materials: Template Synthesis, Structure, and Applications Masanori Yamamoto*, Shunsuke Goto, Rui Tang, and Kaoru Yamazaki* Chem. Sci. 2024, 15, 1953‒1965 DOI: 10.1039/d3sc05022j (Corresponding Author) |
 |
Porous Nanographene Formation on γ‒Alumina Nanoparticles via Transition-Metal-Free Methane Activation Masanori Yamamoto*, Qi Zhao, Shunsuke Goto, Yu Gu, Takaaki Toriyama, Tomokazu Yamamoto, Hirotomo Nishihara, Alex Aziz, Rachel Crespo-Otero, Devis Di Tommaso*, Masazumi Tamura, Keiichi Tomishige, Takashi Kyotani, and Kaoru Yamazaki* Chem. Sci. 2022, 13, 3140‒3146 DOI: 10.1039/d1sc06578e |
Graphene is a carbon material made from planarly arranged hexagons, showing notable physical properties.[1] When pentagons are introduced to the plain, fullerene as a 0-dimensional sphere could be obtained with a positive curvature.[2] When wrapped cylindrically, carbon nanotubes as 1-dimensional materials could be obtained.[3] On the contrary, introduction of heptagons and octagons into the graphene architecture will lead to a non-planar structure with a negative curvature, and the presence of the corresponding ordered 3-dimensional graphene materials has been predicted by Mackay and co-workers in 1992.[4] Figure 1A shows the schematic of Minimal Surface Graphene.
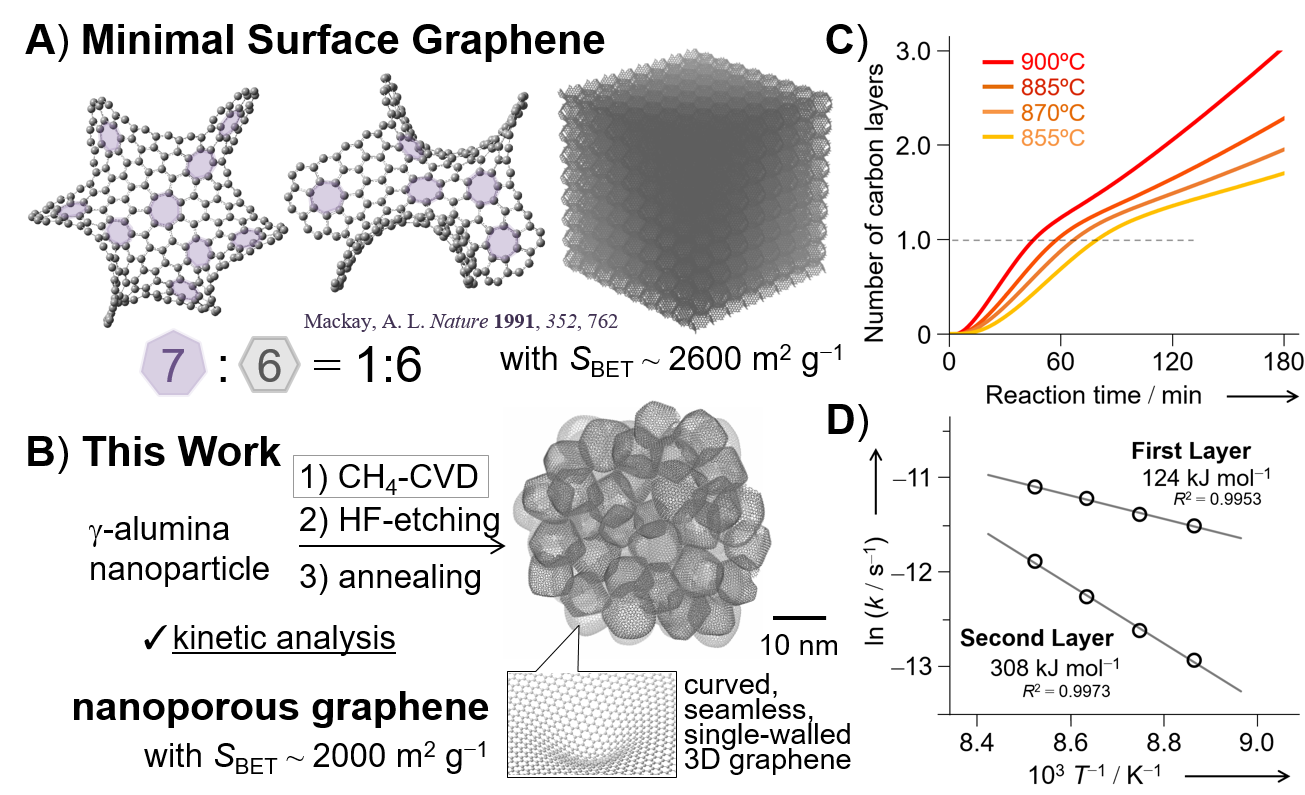
Figure 1. A) Schematics of 3D graphenes, and B‒D) kinetic analysis of CH4 activation on metal oxide nanoparticles.
However, the synthesis of such an ideal 3D graphene material has yet to be achieved: Many research groups have approached to this ideal material, and one of the state-of-the-art material would be zeolite-templated carbon (ZTC): Prof. Takashi Kyotani in Tohoku University reported the synthesis of ZTC by chemical vapor deposition (CVD) of propylene and other hydrocarbons,[5] and ZTC showed an ordered structure of the unit cell with 1.2 nm periodicity as confirmed by XRD, which reflected the ordered structure of the corresponding zeolite template. However, detailed analysis of ZTC including Raman spectroscopy and temperature-programmed desorption of gases showed that ZTC is amorphous carbon with many edge-defects rather than "graphene." On the contrary, Dr. Takahiro Morishita, Prof. Michio Inagaki, and co-workers reported that the use of MgO as a template of CVD-based carbonization led to high-quality porous graphene materials.[6] Later, we also reported that chemically stable methane (CH4) rather than reactive gases including acetylene[7] would be deposited onto the surfaces of alumina nanoparticles (Figure 1B) to give high-quality nanoporous graphene (NPG),[8] with fascinating physicochemical features including excellent electrochemical properties owing to the continuous 3D graphene architecture as confirmed by Raman spectroscopy.[8]
A structural regularity of ZTC and a high-quality of graphene (NPG) should be achieved at the same time for the synthesis of "minimal surface porous graphene materials," but currently we have no template materials that can tolerate high-temperature CH4-CVD: We recognized that CH4-CVD at high temperatures led to the thermal collapse of the structures when many conventional templates were tested. If we reduce the reaction temperature of CH4-CVD by enhancing the reaction rate, the synthesis of "minimal surface porous graphenoid materials" could be ensured using the present template materials available. To this aim, we investigated detailed surface chemistry and kinetics during CH4-CVD on alumina nanoparticles, to elucidate the key factor governing the CH4-CVD reaction.
Practically, the kinetic analysis of CH4-CVD on γ‒alumina nanoparticles at various temperatures (Figure 1C,D) was coupled with computational chemistry using density functional theory (DFT) to elucidate the surface chemistry. As a consequence, we found the followings:
A) |
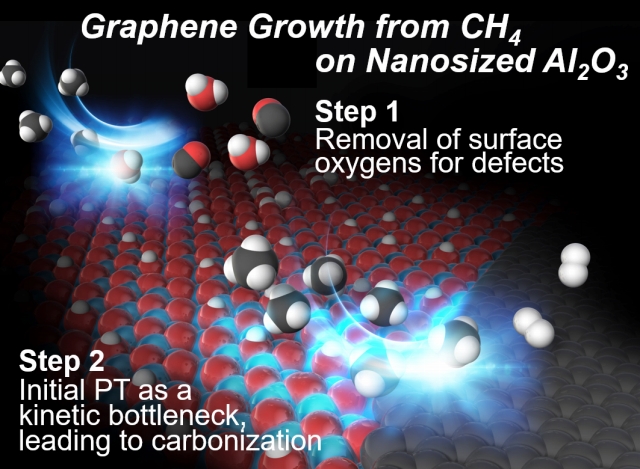
CH4-CVD was initiated at 900°C following the formation of surface oxygen defect by reactions with CH4. |
B) |
The formed surface defect catalyzes the early-stage CH4 activation with no use of transition metal reaction center, and this is supported by both experimental and computational chemistry. |
C) |
Kinetic analysis showed that the rate of CH4-CVD is pseudo-first order with respect to CH4 partial pressure, and the apparent activation energy of CH4-CVD (Ea‡) was 124 kJ mol‒1 for the first-layer deposition (Figure 1D). This value is in a good agreement with an apparent activation energy of the initial dissociative adsorption of CH4 on the metal oxide surface (Ea‡ = 120 kJ mol‒1) calculated by DFT method. |
D) |
The kinetic analysis indicated that the rate-determining step of the CH4-CVD is the initial associative adsorption of CH4, and charge density analysis by DFT calculation suggested that the rate-determining step is a proton transfer (PT) from a CH4 molecule by a conventional Lewis acid/base mechanism[9] rather than radical mechanism.

Figure 2. Energy level diagram for methane activation on a gamma-alumina surface having a surface defect generated upon the reaction with methane molecules. Details are discussed in the paper.
|
E) |
Sequential PTs from CH4 on surfaces of metal oxides gave a reactive surface-bound methylene (Al=CH2*), and its dimerization and subsequent couplings will lead to carbonization on surfaces.[10] |
F) |
Due to the catalysis of defected surfaces, the carbonization on metal oxide nanoparticles (Ea‡ = 124 kJ mol‒1) is more than 2-times faster than the carbonization of deposited carbon (Ea‡ = 308 kJ mol‒1), and this kinetically enabled us to synthesize single-walled porous nanographene. |
Thus, activation of methane on alumina nanoparticles was kinetically feasible to give porous nanographene (NPG) materials with no use of transition metal active centers by CH4-CVD conditions, and the early-stage activation of CH4 has been supported by both experimental and computational chemistry.[10,11] This study shows that a surface oxygen defect formed by high-temperature reaction with CH4 plays a crucial role in CH4-CVD. Activation of chemically stable CH4 was initiated by the single proton transfer (PT) step, and the PT from CH4 is the rate-limiting step of the whole process in CH4-CVD. Advanced surface engineering for defects will improved the reactivity to CH4 and lowering the reaction temperature of CH4 activation on surfaces of metal oxides, and this will enable us to synthesizing "ideal 3D nanoporous graphenes" by CH4-CVD using readily available templates having ordered nanoporosity in the future.[12]
This work was supported by Grants-in-Aid (19K15281) from Japan Society for the Promotion of Science (JSPS), the Ebara Hatakeyama Memorial Foundation, and Ensemble Grant for Early Career Researchers at Tohoku University.
Last modified on 4th April 2025
Keywords
| CH4 activation direct methane reforming nanoporous graphene chemical vapour deposition DFT |
Contact Information
|
Assist. Professor of Chemical Science |
References
| 1. | K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva, A. A. Firsov, Science 2004, 306, 666 |
| 2. | H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl, R. E. Smalley, Nature 1985, 318, 162‒163 |
| 3. | a) S. Iijima, T. Ichihashi, Nature 1993, 363, 603‒605; b) S. lijima, T. Ichihashi, Y. Ando, Nature 1992, 356, 776‒778; c) P. M. Ajayan, S. Iijima, Nature 1992, 358, 23 |
| 4. | H. Terrones, A. L. Mackay, Nature 1991, 352, 762‒763 |
| 5. | Z. Ma, T. Kyotani, A. Tomita, Chem. Commun. 2000, 2365‒2366 |
| 6. | a) M. Inagaki, S. Kobayashi, F. Kojin, N. Tanaka, T. Morishita, B. Tryba, Carbon 2004, 42, 3153‒3158; b) T. Morishita, T. Tsumura, M. Toyoda, J. Przepiorski, A. W. Morawski, H. Konno, M. Inagaki, Carbon 2010, 48, 2690‒2707; c) M. Inagaki, M. Toyoda, Y. Soneda, S. Tsujimura, T. Morishita, Carbon 2016, 107, 448‒473 |
| 7. | Y. Tian, X. Zhu, M. Abbas, D. W. Tague, M. A. Wunch, J. P. Ferraris, K. J. Balkus, ACS Appl. Energy Mater. 2022, 5, 6805‒6813 |
| 8. | a) K. Yamazaki, S. Goto, S. Yoshino, A. Gubarevich, K. Yoshida, H. Kato, M. Yamamoto, Phys. Chem. Chem. Phys. 2023, 25, 32972‒32978; b) M. Yamamoto, S. Goto, R. Tang, K. Nomura, Y. Hayasaka, Y. Yoshioka, M. Ito, M. Morooka, H. Nishihara, T. Kyotani, ACS Appl. Mater. Interfaces 2021, 13, 38613‒38622; c) S. Sunahiro, K. Nomura, S. Goto, K. Kanamaru, R. Tang, M. Yamamoto, T. Yoshii, J. N. Kondo, Q. Zhao, A. G. Nabi, R. Crespo-Otero, D. Di Tommaso, T. Kyotani, H. Nishihara, J. Mater. Chem. A 2021, 9, 14296‒14308; d) H. Nishihara, T. Simura, S. Kobayashi, K. Nomura, R. Berenguer, M. Ito, M, Uchimura, H. Iden, K. Arihara, A. Ohma, Y. Hayasaka, T. Kyotani, Adv. Funct. Mater. 2016, 26, 6418‒6427 |
| 9. | A. Matsuda, H. Tateno, K. Kamata, M. Hara, Catal. Sci. Technol. 2021, 11, 6987‒6998 |
| 10. | Q. Zhao, M. Yamamoto, K. Yamazaki, H. Nishihara, R. Crespo-Otero, D. Di Tommaso, Phys. Chem. Chem. Phys. 2022, 24, 23357‒23366 |
| 11. | M. Yamamoto, Q. Zhao, S. Goto, Y. Gu, T. Toriyama, T. Yamamoto, H. Nishihara, A. Aziz, R. Crespo-Otero, D. Di Tommaso, M. Tamura, K. Tomishige, T. Kyotani, K. Yamazaki, Chem. Sci. 2022, 13, 3140‒3146 (DOI: 10.1039/d1sc06578e) |
| 12. | M. Yamamoto, S. Goto, R. Tang, K. Yamazaki, Chem. Sci. 2024, 15, 1953‒1965 (DOI: 10.1039/d3sc05022j) |
